ELECTRICITY AND CHEMICAL REACTIONS
Introduction
The properties of an element or a compound are associated with its atomic structure. Physical and chemical properties can be explained on the basis of atomic structure. This is why scientists study atomic structure in physics and in chemistry. Therefore both in Physics and Chemistry scientists study atomic structure. Electricity is the flow of electrons and that when electrons are gained or lost, the chemical structure of a substance changes. Hence, how chemical reactions and electricity are related is a separate field of study in chemistry. Whether a substance conducts or resists electricity is related to its atomic structure. The response of chemicals to electricity is studied in chemistry. Electricity is used in large and small industries and in the manufacture of many chemicals and it is of importance to chemistry.
Electrochemistry is the study of reactions between electricity and chemical substances. There are various instances of chemical changes producing electrical energy and vice versa. Electrochemistry deals with reactions in which electrical energy and chemical energy are mutually transformed. Electrochemical cells are set-arrangements where this energy conversion takes place.
Galvanic Cells
If we observe the changes that happen to copper sulphate solution in which a zinc strip is immersed we can see change in the colour of the solution.
The reaction which takes place here is:
Zn⇒Zn2+ + 2e_
Cu2+ (SO4)2- + 2e_ ⇒Cu + SO42_
Zn + CuSO4 ⇒Zn2+SO42_ + Cu
Here, we can find which reaction is oxidation and which reaction is reduction.
Now, immerse Zn strip in ZnSO4 solution and a Cu strip in CuSO4 solution. Observe the changes the solutions undergo.
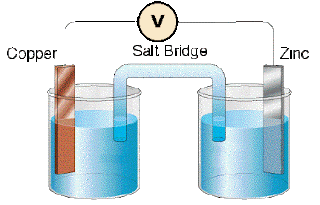
Conduct the following experiment. Take ZnSO4 solution in a beaker and immerse a Zn strip in it. In another beaker take CuSO4 solution and immerse a Cu strip in it. Connect the electrodes with a voltmeter through a conductor.
Now, cut a strip of filter paper and soak it in a solution of potassium nitrate. Using the soaked filter paper, connect the two solutions. There will be a continuous flow of electricity now. When a metal is immersed in a solution of its own ion, it displays a tendency to form ions by losing electrons. At the same time, metal ions in the solution show the tendency to form metal atom by accepting the electrons. This can be represented by the following equations.
I. Zn electrode : Zn ⇒Zn2+ + 2e-
ZnSO4 solution : Zn2+ + 2e- ⇒Zn
II Cu electrode : Cu ⇒Cu2+ + 2e- ⇒Cu
Zinc gives two electrons to the electrode that each Zn atom dissolves. At the same time Zn2+ ions have the tendency to become Zn atom. Due to the opposing tendencies, an electrical potential develops at the place where the electrons from the Zn electrode join an equal number of Zn2+ that arrive at that place. This is called electrode potential and is indicated by the letter E.
To represent the potential of zinc electrode we write EZn2+/Zn and for that of Cu electrode, we write ECu2+/Cu.
For electric flow to take place when zinc and copper are connected, their electrode potentials must be different.
Galvanic cells are instruments that convert chemical energy into electrical energy.
Anode and Cathode
The electrode which oxidation takes place is called the anode and the one at which reduction takes place is called the cathode.
Electrons go from Zn electrode dipped in ZnSO4 solution to Cu electrode dipped in CuSO4 solution.
Anode | Cathode | Chemical Reaction | |
Anode | Cathode | ||
Zn | Cu | Zn ⇒Zn2+ + 2e- | Cu2+ + 2e- ⇒ Cu |
If expressed in relation to oxidation – reduction hypothesis, when electricity is produced in a galvanic cell, it can be said that oxidation takes place on the side of zinc electrode and reduction takes place on the side of copper electrode.
Cell em.f.
The potential difference between two electrodes is termed as the cell e.m.f. (cell electromotive force).
e.m.f = E2 – E1
Where E1, E2 are the electrode potentials.
To measure this, the Standard Hydrogen Electrode – SHE is taken as the reference. The electrode potential of SHE is taken as ‘zero’ at 298 K, one atm pressure and a concentration of 1 molar (1 M) H+ solution. To find the electrode potential of any particular electrode, it is enough to measure the e.m.f of the cell obtained by connecting that electrode with a standard hydrogen electrode.
If the voltmeter reading obtained by connecting Zn electrode immersed in ZnSO4 solution with a SHE is 0.76, the electrode potential of Zn electrode (EZn2+/Zn) can be taken as 0.76 V.
Electrode | Reaction at the hydrogen electrode | Volt meter reading | Electrode potential |
Zn2+/Zn | Reduction | 0.76 | -0.76 |
Cu2+/Cu | Oxidation | 0.34 | +0.34 |
The value of the electrode potential that we get here can be either positive or negative. It depends on the chemical reactions that take place at the hydrogen electrode. If the reaction at hydrogen electrode is oxidation, this value will be positive and if it is reduction, the value will be negative.
In which of the two electrodes of an electrochemical cell, oxidation or reduction takes place is decided by the value of the electrode potential. Oxidation takes place (anode) at the electrode which has a low potential and reduction (cathode) at the electrode which has a high potential.
E˚cell = E˚Cu2+/Cu - E˚Zn2+/Zn
= (+0.34 V) – (-0.76 V)
= +1.1V
The series in which electrodes are arranged according to electrode potential in the standard state is called electrochemical series.
Electrolysis
Compounds that decompose into oppositely charged particles while in aqeous solution or in molten state are called electrolytes. The decomposition of an electrolyte into its ions by the passage of electricity is called electrolysis.
Faraday’s law of electrolysis
When the quantity of electricity is increased, the amount of copper deposited also increases, i.e., they are directly proportional.
The above relationship was first stated by Faraday. Hence it is known as Faraday’s law of electrolysis.
If ‘q’ is the quantity of electricity and ‘m’, the mass of substance deposited then the above relation can be mathematically expressed as m α q
m = a constant x q
= z x q
‘z’ is the amount of substance deposited when one coulomb electricity is allowed to pass through.